A
B
C
D
Text Solution
Verified by Experts
MODERN PUBLICATION-CHEMICAL KINETICS-EXERCISE
- For the reaction, N2+3H2 rarr 2NH3 if (d [NH3])/(dt)=2 xx 10^-4 mol//L...
Text Solution
|
- In the reaction BrO3^(-)(aq) + 5Br^(-) + 6H^(+) rarr 3Br2 + 3H2O the r...
Text Solution
|
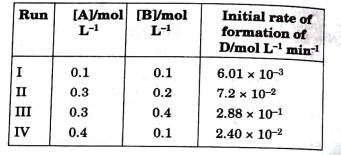
- During the kinetic study of the reaction. 2A + B rarr C + D, followin...
Text Solution
|
- For the reaction N2O5(g)rarr 2NO2(g) + 1/2 O2 (g) the value of rate of...
Text Solution
|
- In a reaction, A + B rarr Product, rate is doubled when the concentrat...
Text Solution
|
- In a zero-order reaction for every 10^@ rise of temperature, the rate ...
Text Solution
|
- What is the activation energy for a reaction if its rate double when t...
Text Solution
|
- The rate constant of the reaction A rarr B is 0.6 xx10^-3 mole per sec...
Text Solution
|
- The activation energy of a reaction can be determined by
Text Solution
|
- When initial concentration of a reactant is doubled in a reaction, its...
Text Solution
|
- The addition of a catalyst during a chemical reaction alters which of ...
Text Solution
|
- The rate of first-order reaction is 0.04 mol L^-1 s^-1 at 10 seconds a...
Text Solution
|
- The decomposition of phosphine (PH3) on tungsten at low pressure is a ...
Text Solution
|
- What is the time required for a first order reaction to be 99 % comple...
Text Solution
|
- The experimental rate law for a reaction : 2A + B rarr Product is rat...
Text Solution
|
- If the initial concentration of the reactant is doubled, the time for ...
Text Solution
|
- For a reaction taking place in three steps, the rate constants are k1,...
Text Solution
|
- For a zero order reaction, the plot of concentration of reactant vs ti...
Text Solution
|
- Consider the following statements : (i) increase in concentration of ...
Text Solution
|
- The initial rates of reaction E 3A + 2B + C rarr Products, at differen...
Text Solution
|