A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL KINETICS-EXERCISE
- For a zero order reaction, the plot of concentration of reactant vs ti...
Text Solution
|
- Consider the following statements : (i) increase in concentration of ...
Text Solution
|
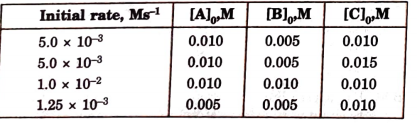
- The initial rates of reaction E 3A + 2B + C rarr Products, at differen...
Text Solution
|
- For a reaction A + B rarr C+2D, experimental results were collected fo...
Text Solution
|
- In a first order reaction, the concentration of the reactant is reduce...
Text Solution
|
- The activation energy for the reaction if k = A is
Text Solution
|
- The decomposition of ammonia on tungsten surface at 500 K follows zero...
Text Solution
|
- The rate constant of a first order reaction is doubled when the temper...
Text Solution
|
- The rate of a reaction is given by rate, r = k [H^+]^n If the rate bec...
Text Solution
|
- In a first order reaction, 80 % of the reactant at an instant was redu...
Text Solution
|
- The decomposition of N2O5 in CCl4 solution at 318 K is studied by moni...
Text Solution
|
- In a first order reaction, the concentration of the reactant decreases...
Text Solution
|
- The following mechanism has been proposed for the reaction of NO and B...
Text Solution
|
- Rate of a reaction can be expressed by Arrhenius equation as: k= Ae^(-...
Text Solution
|
- Consider the reaction, 2A + B rarr Products When concentration of B al...
Text Solution
|
- The half life period of a first order reaction is 6.93 minutes. The ti...
Text Solution
|
- The time for half life period of a certain reaction A rarr Products i...
Text Solution
|
- The rate of chemical reaction double for every 10^@Сrise of temperatur...
Text Solution
|
- For a first order reaction, (A) rarr Products, the concentration of A ...
Text Solution
|
- The rate of a reaction doubles when its temperature changes from 300 K...
Text Solution
|