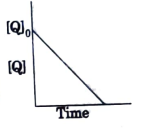
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL KINETICS-EXERCISE
- State the number of electrons present in the outermost shell of the at...
Text Solution
|
- Fill in the blanks- is an alloy used foe making aircrafts and aeroplan...
Text Solution
|
- In the reaction, P+Q rarr R +S+the time taken for 75% reaction of P is...
Text Solution
|
- The initial rate of hydrolysis of methyl acetate (1M) by a weak acid (...
Text Solution
|
- For the elementary reaction M rarr N, the rate of disappearance of M i...
Text Solution
|
- If rate of reaction in terms of disappearance of NH3 is -(d[NH3])/(dt)...
Text Solution
|
- Consider the rate law expression for a reaction : rate = k[NO2^-] [I^-...
Text Solution
|
- For a first order reaction :
Text Solution
|
- In acidic medium, the rate of reaction between BrO3^- and Br^- is give...
Text Solution
|
- The rate law for the reaction : RCl + NaOH rarr ROH + NaCl is given ...
Text Solution
|
- Which of the following statements is not correct. Some antisecptics ca...
Text Solution
|
- Fill in the blanks- is an mixture which is used as domestic fuel.
Text Solution
|
- For the first order reaction 2N2O5 (g) rarr 4 NO2(g)+ O2(g) which is i...
Text Solution
|
- Consider the following reaction for 2NO2(g) + F2(g) rarr 2NO2F(g). The...
Text Solution
|
- Arrhenius equation is
Text Solution
|
- The integrated rate equations can be fitted with kinetic data to deter...
Text Solution
|
- The integrated rate equations can be fitted with kinetic data to deter...
Text Solution
|
- The integrated rate equations can be fitted with kinetic data to deter...
Text Solution
|
- The integrated rate equations can be fitted with kinetic data to deter...
Text Solution
|
- For a zero order reaction, linear plot was obtained for [A] vs t. The ...
Text Solution
|