A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL KINETICS-EXERCISE
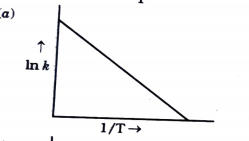
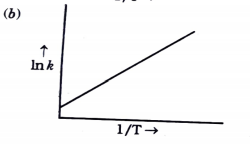
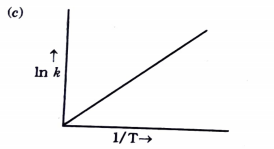
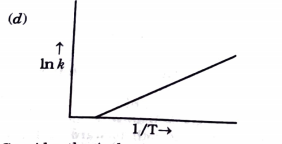
- Consider the following figure and mark the correct option.
Text Solution
|
- Consider a first order gas phase decomposition reaction given below : ...
Text Solution
|
- According to Arrhenius equation rate constant k is equal to A e^(-Ea//...
Text Solution
|
- Consider the Arrhenius equation given below and mark the correct optio...
Text Solution
|
- A graph of volume of hydrogen released Vs time for the reaction betwee...
Text Solution
|
- Which of the following statements is not correct about order of a reac...
Text Solution
|
- Consider the graph given in previous question. Which of the following ...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Which of the following graphs represents exothermic reaction?
Text Solution
|
- Rate law for the reaction A + 2B to C is found to be rate = k [A][B]...
Text Solution
|
- Which of the following statements is incorrect about the collision the...
Text Solution
|
- A first order reaction taken 16 minutes for 50% completion. How much t...
Text Solution
|
- Compounds ‘A’ and ‘B’ react according to the following chemical equati...
Text Solution
|
- Which of the following statements are not correct regarding rate of ca...
Text Solution
|
- Define the following terms : Pseudo first order reaction.
Text Solution
|
- Consider the reaction A harr B. The concentration of both the reactant...
Text Solution
|
- In the following questions two or more options may be correct. Rate la...
Text Solution
|
- Which of the following statements are applicable to a balanced chemica...
Text Solution
|
- What is the maximum number of electrons that can go into the N shell o...
Text Solution
|
- What is the electronic configuration of a hydrogen atom ?
Text Solution
|