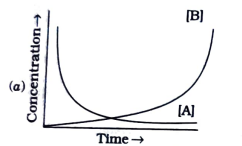
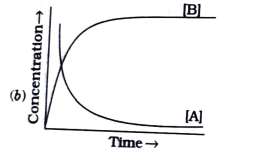
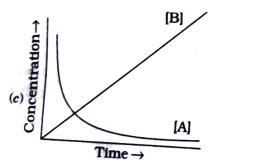
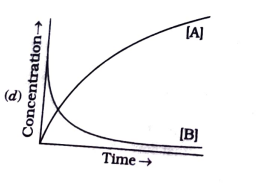
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL KINETICS-EXERCISE
- Which of the following statements are not correct regarding rate of ca...
Text Solution
|
- Define the following terms : Pseudo first order reaction.
Text Solution
|
- Consider the reaction A harr B. The concentration of both the reactant...
Text Solution
|
- In the following questions two or more options may be correct. Rate la...
Text Solution
|
- Which of the following statements are applicable to a balanced chemica...
Text Solution
|
- What is the maximum number of electrons that can go into the N shell o...
Text Solution
|
- What is the electronic configuration of a hydrogen atom ?
Text Solution
|
- At high pressure the following reaction is zero order. 2NH3(g) overset...
Text Solution
|
- During decomposition of an activated complex
Text Solution
|
- According to Maxwell Boltzmann distribution of energy, ,
Text Solution
|
- Name the subatomic particle whose relative charge is : (b) –1
Text Solution
|
- Which of the following statements are in accordance with the Arrhenius...
Text Solution
|
- Fill in the blanks- ,, are the things that are made of wood.
Text Solution
|
- Which of the following graphs is correct for a zero order reaction?
Text Solution
|
- Name the subatomic particle whose relative charge is : (c) 0
Text Solution
|
- Complete the reaction
Text Solution
|
- Which shell of an atom can accommodate a maximum of : (a) 8 electrons ...
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Which shell of an atom can accommodate a maximum of : (b) 32 electron...
Text Solution
|
- Name the shell of an atom which can accommodate a maximum of : (a) 2 e...
Text Solution
|