A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SURFACE CHEMISTRY-EXERCISE
- The correct statement(s) pertaining to the adsorption of a gas on a so...
Text Solution
|
- The mixture of one parts of concentrated nitric acid and three parts o...
Text Solution
|
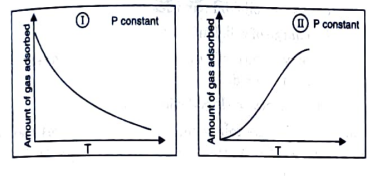
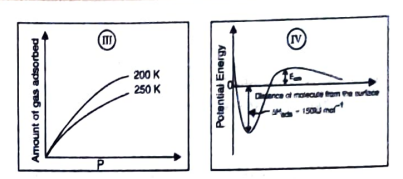
- The given graphs/data I, II, III and IV represent general trends obser...
Text Solution
|
- When O2 is adsorbed on a metallic surface, electron transfer occurs fr...
Text Solution
|
- The size of colloidal particles is
Text Solution
|
- Which mixture is used to dissolve precious metal gold?
Text Solution
|
- Fill in the blanks with appropriate answer- Mixture of conc. nitric ac...
Text Solution
|
- State whether the statement is true or false- Mixture of concentrated ...
Text Solution
|
- Explain the following terms-Aquaregia.
Text Solution
|
- Fill in the blanks- Gold metal can be dissolved only in .
Text Solution
|
- Fill in the blanks- Good quality electric wires are made up of alloy.
Text Solution
|
- Which of the following electrolyte requires maximum concentration to c...
Text Solution
|
- The nucleus of an atom has 5 protons and 6 neutrons. What would be the...
Text Solution
|
- The nucleus of an atom has 5 protons and 6 neutrons. What would be the...
Text Solution
|
- Assertion : Small quantity of soap is required to prepare a stable emu...
Text Solution
|
- Assertion : Sea water looks blue. Reason : Due to scatting of light b...
Text Solution
|
- Write the electronic configuration of the element with atomic number 1...
Text Solution
|
- The atomic number of an element X is 16. (a) Write down the electronic...
Text Solution
|
- The atomic number of an element X is 16. (b) What will be the valency...
Text Solution
|
- What is the reason for the different atomic masses of the isotopes of ...
Text Solution
|