Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SURFACE CHEMISTRY-EXERCISE
- What is the reason for the different atomic masses of the isotopes of ...
Text Solution
|
- State whether the statement is true or false- Phosgene is the commerci...
Text Solution
|
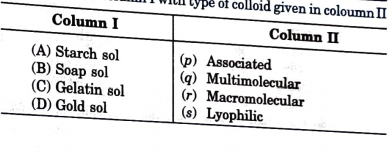
- Match the column I with type of colloid given in coloumn II.
Text Solution
|
- Match list I with list II and select the correct answer using the code...
Text Solution
|
- Match list I of enzymatic reaction with enzyme given in list II:
Text Solution
|
- An element has Z = 7. What is the valency of the element ? Also name t...
Text Solution
|
- What is the number of valence electrons in the atoms of an element hav...
Text Solution
|
- Give the number of protons, neutrons and electrons in the chlorine ha...
Text Solution
|
- The mass number of two atoms X and Y is the same (40 each) but their a...
Text Solution
|
- Elements having valency ‘one’ are :
Text Solution
|
- For an element, Z = 9. The valency of this element will be :
Text Solution
|
- The number of valence electrons in a sulphide ion, S2–, is :
Text Solution
|
- The atomic number of an element X is 8 and that of element Y is 4. Bot...
Text Solution
|
- What is the number of valence electrons in : (a) sodium ion, Na+
Text Solution
|
- In physisorption adsorbent does not show specificity for any particula...
Text Solution
|
- Which of the following is an example of absorption ?
Text Solution
|
- On the basis of data given below predict which of the following gases ...
Text Solution
|
- In which of the following reactions heterogenous catalyse is involved?...
Text Solution
|
- What is the number of valence electrons in : (b) oxide ion, O2–
Text Solution
|
- Atom A has a mass number 209 and atomic number 82. Atom B has a mass n...
Text Solution
|