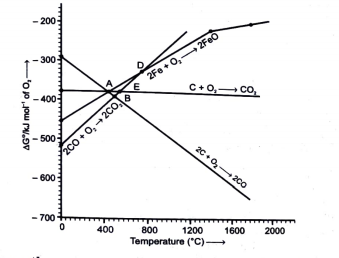
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS-EXERCISE
- Electrolytic refining is used to purify which of the following metals ...
Text Solution
|
- Extraction of gold and silver involves leaching the metal with CN^- io...
Text Solution
|
- Answer the question on the basis of figure given below : Choose th...
Text Solution
|
- Answer the question on the basis of figure given below : Below poi...
Text Solution
|
- Answer the question on the basis of figure given below : For the r...
Text Solution
|
- In the following questions two or more options may be correct. At the ...
Text Solution
|
- In the following questions two or more options may be correct. Which o...
Text Solution
|
- In the extraction of aluminium by Hall-Heroult process, purified Al2O3...
Text Solution
|
- Which of the following statements is correct ?
Text Solution
|
- In the Froth Floatation process, zinc sulphide and lead sulphide can b...
Text Solution
|
- Common impurities present in bauxite are .
Text Solution
|
- Which of the following ores are concentrated by froth floation ?
Text Solution
|
- Which of the following reactions occur during calcination?
Text Solution
|
- For the metallurgical process of the ores calcined ore can be reduced ...
Text Solution
|
- The main reactions occurring in blast furnace during extraction of iro...
Text Solution
|
- In which of the following method of purification, metal is converted t...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- Give the preparation of chlorine.
Text Solution
|
- Match the items of Column I with items of Column II and assign the cor...
Text Solution
|
- Match the items of Column I with items of Column II and assign the cor...
Text Solution
|