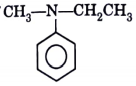
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN -EXERCISE
- The strongest base is :
Text Solution
|
- Benzylamine react with nitrous acid to form.
Text Solution
|
- The IUPAC name of is
Text Solution
|
- Gabriel phthalimide reaction is used for the preparation of
Text Solution
|
- Which of the following compound will be formed when aniline reacts wit...
Text Solution
|
- Which one of the following is most basic ?
Text Solution
|
- C2H5NH2 +HNO2 rarr A, A is :
Text Solution
|
Text Solution
|
- Which among the following compound will give offensive compound when h...
Text Solution
|
- 3^@ amines do not undergo acylation why ?
Text Solution
|
- Explain the following : CH3NH2 is stronger base than ammonia.
Text Solution
|
- Why is it difficult topreparepure amines by Hofmann’s ammonolysis?
Text Solution
|
- An amine (A) C3H9N reacts with nitrous acid at 0 to 5^@ C to give an o...
Text Solution
|
- An amine (A) C3H9N reacts with nitrous acid at 0 to 5^@ C to give an o...
Text Solution
|
- Why have primary amines higher boiling point than tertiary amines?
Text Solution
|
- How can you find out whether a given amine is a primary amine ? Write ...
Text Solution
|
- In the following cases rearrange the compounds as directed: In an incr...
Text Solution
|
- Arrange the following : Increasing order of basic strength : Aniline, ...
Text Solution
|
- Arrange the following : In decreasing order of the pKb values: C2H5NH2...
Text Solution
|
- Complete the following chemical equations : C6H5N2Cl+C6H5NH2 overset...
Text Solution
|