MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN -EXERCISE
- Complete the following chemical equations : CH3CH2NH2+CHCl3+alc. KOH...
Text Solution
|
- Complete the following chemical equations : C6H5N2^+Cl^(-) overset ...
Text Solution
|
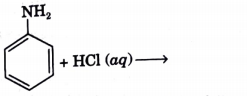
- Complete the following reaction :
Text Solution
|
- Complete the following reactions : C6H5N2Cl+H3 PO2+H2O rarr
Text Solution
|
- Write the main products of the following reactions -
Text Solution
|
- Write the main products of the following reactions - CH3- underset (O)...
Text Solution
|
- Write the structures of products in the following : CH3CH2 NH2 overset...
Text Solution
|
- Write the structure of the product obtained in the following :
Text Solution
|
- Write the structures of products in the following :
Text Solution
|
- Write the structures of A, B and C in the following reaction : C6H5 CO...
Text Solution
|
- Write the structures of A, B and C in the following reaction : C6H5N2^...
Text Solution
|
- Why aniline a weaker base than methylamine?
Text Solution
|
- How is the activation of benzene ring by amino group reduced by acetyl...
Text Solution
|
- How is sulphanilic acid prepared from aniline ?
Text Solution
|
- What happens when aniline reacts with K2Cr2O7 and H2SO4.
Text Solution
|
- How are amines prepared from nitro alkanes?
Text Solution
|
- How are amines prepared from alkyl halide?
Text Solution
|
- How are amines prepared from amides?
Text Solution
|
- How does benzene diazonium chloride react with : Water
Text Solution
|
- How does benzenediazonium chloride react with : HNO2
Text Solution
|