Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN -EXERCISE
- Discuss Coupling reaction of benzenediazonium chloride.
Text Solution
|
- Aniline does not undergo Friedel-Crafts reaction. Explain.
Text Solution
|
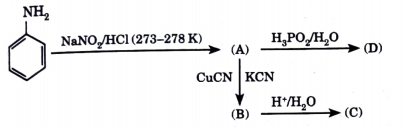
- Identify the compounds (A) (B), (C), (D) in the following sequence of...
Text Solution
|
- Why are aliphatic amines more basic than aromatic amines?
Text Solution
|
- Why aromatic amines cannot be prepared by Gabriel phthalimide synthesi...
Text Solution
|
- Give reason for the following statement- Mixture of 1% of phosphorus, ...
Text Solution
|
- Write short notes on the followng : Hoffmann’s bromamide reaction.
Text Solution
|
- Why is aniline less basic than ethylamine ?
Text Solution
|
- Complete the following reactions : C6H5NH2 +CHCl3 +alc. KOH rarr
Text Solution
|
- Explain the following : Arrange the following in increasing order of b...
Text Solution
|
- Write short notes on the followng : Coupling reaction
Text Solution
|
- Explain the following : Ammonia is less basic than methylamine. Why?
Text Solution
|
- Explain the following : Complete the following reaction: C6H5N2Cl + H3...
Text Solution
|
- Arrange the following : Decreasing order of basic strength in gas phas...
Text Solution
|
- Explain the following : Carbylammine reaction.
Text Solution
|
- Why ethylamine is more basic than methyl amine.
Text Solution
|
- Explain the following : Complete the following reaction: C6H5NH2 + Br2...
Text Solution
|
- Arrange the following in decreasing order of their basic strength in a...
Text Solution
|
- An aromatic compound ‘A’ of molecular formula C7H7ON undergoes a serie...
Text Solution
|
- Write the structures of main products formed when aniline reacts with ...
Text Solution
|