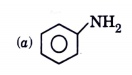
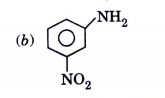
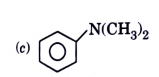
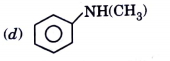
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN -EXERCISE
- Bromobenzene can be prepared when benzene diazonium chloride is treate...
Text Solution
|
- 2X+B2H6 rarr [BH2(X)2]^+ [BH4]^- The amine(s) X is/are
Text Solution
|
- Amines are basic in nature due to the presence of lone pair of electro...
Text Solution
|
- Which of the following statement is not correct ?
Text Solution
|
- Maximum pKb value is of
Text Solution
|
- The strongest base among the following is
Text Solution
|
- Which of the following group does not decrease the basic strength of a...
Text Solution
|
- Treatment of compound O with KMnO4//H^+ gave P, which on heating with ...
Text Solution
|
- Treatment of compound O with KMnO4//H^+ gave P, which on heating with ...
Text Solution
|
- The questions given below consist of an Assertion and a Reason. Use th...
Text Solution
|
- Assertion: Aniline does not undergo Friedel Crafts reaction. Reason : ...
Text Solution
|
- Aniline is less basic than ammonia
Text Solution
|
- Write short notes on the followng : Carbylamine reaction.
Text Solution
|
- 3^@ amines do not undergo acylation why ?
Text Solution
|
- Assertion: Aniline hydrogen sulphate, on heating forms a mixture of or...
Text Solution
|
- The reaction between alkyl halides and sodium metal is called :
Text Solution
|
- Sulphanilic acid is insoluble in water and organic solvents. Explain.
Text Solution
|
- In general, alkyl halides are more reactive than aryl halides.
Text Solution
|
- Assertion: p-nitroaniline is a stronger base than p-toluidine. Reaso...
Text Solution
|
- Each question contains statements given in two columns, which have to ...
Text Solution
|