A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN -EXERCISE
- Which of the following group does not decrease the basic strength of a...
Text Solution
|
- Treatment of compound O with KMnO4//H^+ gave P, which on heating with ...
Text Solution
|
- Treatment of compound O with KMnO4//H^+ gave P, which on heating with ...
Text Solution
|
- The questions given below consist of an Assertion and a Reason. Use th...
Text Solution
|
- Assertion: Aniline does not undergo Friedel Crafts reaction. Reason : ...
Text Solution
|
- Aniline is less basic than ammonia
Text Solution
|
- Write short notes on the followng : Carbylamine reaction.
Text Solution
|
- 3^@ amines do not undergo acylation why ?
Text Solution
|
- Assertion: Aniline hydrogen sulphate, on heating forms a mixture of or...
Text Solution
|
- The reaction between alkyl halides and sodium metal is called :
Text Solution
|
- Sulphanilic acid is insoluble in water and organic solvents. Explain.
Text Solution
|
- In general, alkyl halides are more reactive than aryl halides.
Text Solution
|
- Assertion: p-nitroaniline is a stronger base than p-toluidine. Reaso...
Text Solution
|
- Each question contains statements given in two columns, which have to ...
Text Solution
|
- Fill in the blanks- is the commercial name of NaHCO3.
Text Solution
|
- Baking soda is used for-.
Text Solution
|
- Fill in the blanks- is the chemical compound which is used in the deod...
Text Solution
|
- Fill in the blanks- is the chemical compound which is used as teeth w...
Text Solution
|
- The number of isomeric amines of molecular formula C4H11N which give c...
Text Solution
|
- Total number of nitrogen atoms present in reduced product obtained by ...
Text Solution
|
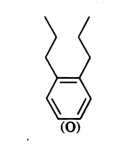 The compound T is
The compound T is