A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KIRAN PUBLICATION-ANALOGY OR SIMILARITY -TYPE-IV (Select the related letter from the given alternatives.)
- Choose the correct answer. ACE:. GIK : : MOQ : ?
Text Solution
|
- EGIK: LJHF:: SUWY:?
Text Solution
|
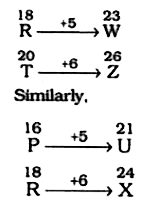
- RT : WZ : : PR : ?
Text Solution
|
- Choose the correct answer. ACF : JLO : : UWZ : ?
Text Solution
|
- BDGH : OQTU : : FHKL : ?
Text Solution
|
- AN : BO : : CP : ?
Text Solution
|
- DDW : ECV : : FBU : ?
Text Solution
|
- BCDZ : CDEV : : DEFT : ?
Text Solution
|
- LO : PK :: IR : ?
Text Solution
|
- QTU : ILM : : BEF : ?
Text Solution
|
- ACEG : ZXVT : : HJLM : ?
Text Solution
|
- AD : NQ : : EH : ?
Text Solution
|
- Choose the correct answer. AZB : BYC : : MVN : ?
Text Solution
|
- BEHI: JMPQ: : KNQR:?
Text Solution
|
- ACFJ : ZXUQ : : DFIM : ?
Text Solution
|
- EFI : IJL: : KLO : ?
Text Solution
|
- ZA: YB:: XC:?
Text Solution
|
- EFG : IJK : : MNO : ?
Text Solution
|
- RAT : TAR : : PIT : ?
Text Solution
|
- Rig : Ofd : : Met : ?
Text Solution
|