Similar Questions
Explore conceptually related problems
Recommended Questions
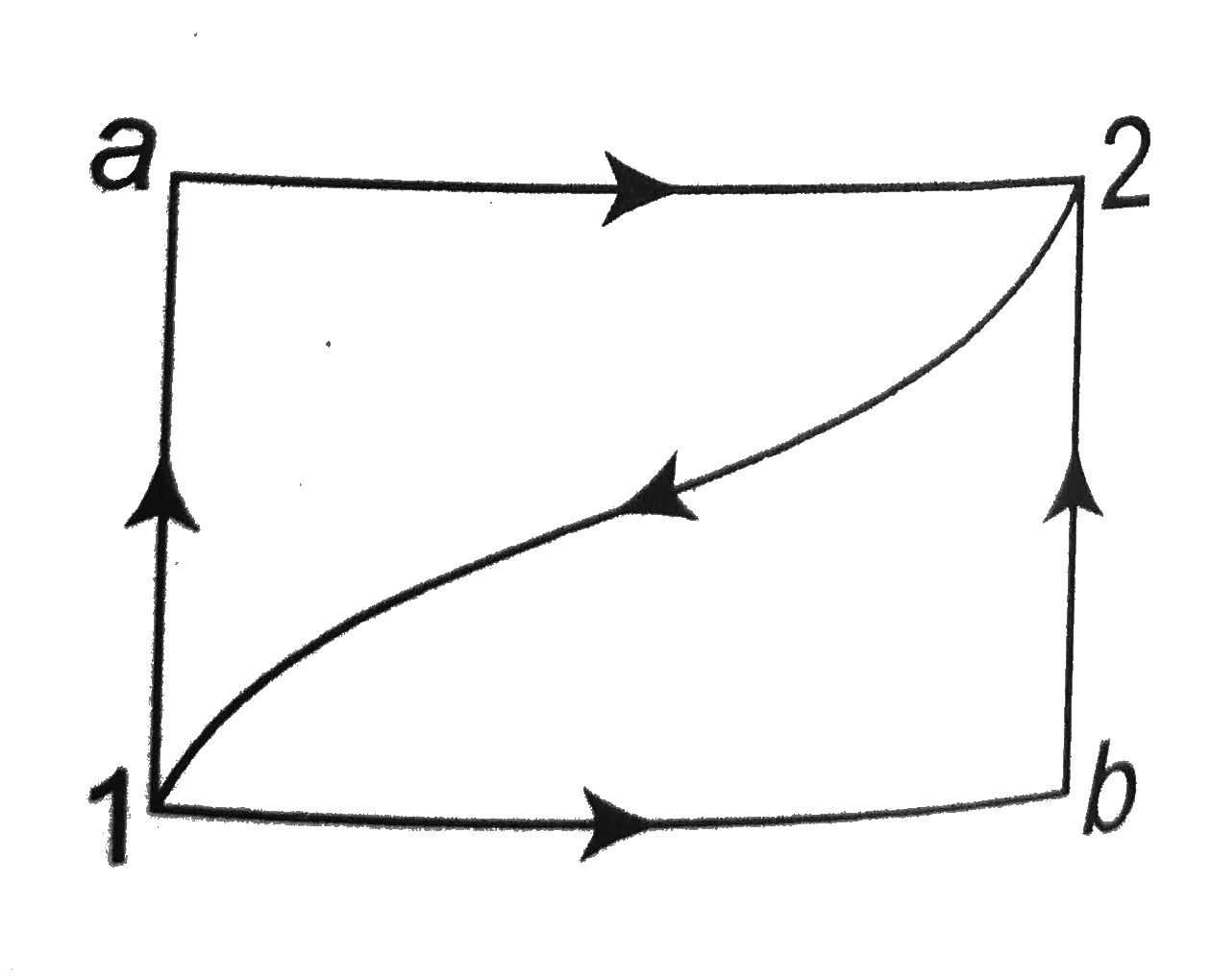
- When a system is taken from state 1 to 2 along the path 1 a 2 it absor...
Text Solution
|
- When a system is taken from state i to state f along the path iaf, it ...
Text Solution
|
- Figure shows an indicator diagram. During path 1-2-3, 100 cal is given...
Text Solution
|
- When a system is taken from state 1 to 2 along the path 1 a 2 it absor...
Text Solution
|
- A system is taken from state a to state c along the path adc (figure)....
Text Solution
|
- When a system is taken from state i to state f alone the path iaf, it ...
Text Solution
|
- A system is taken from state A to state B along two different paths 1 ...
Text Solution
|
- In given figure, when a thermodynamic system is taken from state A to ...
Text Solution
|
- When a system is taken from state A to state B along the path ACB, 80 ...
Text Solution
|