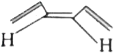
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-QUESTION-PAPERS-2012-Chemistry (Single correct answer type:)
- Which of the following is process used for the preparation of acetone?
Text Solution
|
- Lindane can be obtained by the reaction of benzene with
Text Solution
|
- The structure of cis-bis (propenyl) ethene is
Text Solution
|
- 5 moles of Ba(OH)2 are treated with excess of CO2 . How much BaCO2 wil...
Text Solution
|
- Diatomic molecule has a dipole moment of 1.2D If its bond 1.0 Å what f...
Text Solution
|
- When a gas filled in a closed vessel is heated through 1^(@)C , its p...
Text Solution
|
- For 2NOBr(g) iff 2NO(g) + Br2(g) at equilibrium, P(Br2) = p/q and p is...
Text Solution
|
- The decompostion temperature is maximum for
Text Solution
|
- When the same amount of zinc is treated separately with excess of sulp...
Text Solution
|
- A compound 'X' when reacted with PCI(5) and then with NH(3) gives 'Y'....
Text Solution
|
- Alizarin is an example of
Text Solution
|
- What will be the main product when acetylene reacts with hypochlorous ...
Text Solution
|
- Barium titanate has the pervoskite structure, i.e. a cubinc lattice wi...
Text Solution
|
- Which of the following hormones, is responsible for the growth of anim...
Text Solution
|
- Which of the following has the largest ionic size?
Text Solution
|
- if the radius of H is 0.53 Å then what will be the radius of .(3)Li^(2...
Text Solution
|
- Which of the following will have highest value of pka ?
Text Solution
|
- Gas(A) + NaOH to B overset(Delta)(rarr)Coverset(Delta)(rarr)D C and D ...
Text Solution
|
- The polymer polyurethanes are formed by treating dilsocyanate with
Text Solution
|
- What will be the volume of O(2) Liberated at NTP by passing 5 A curren...
Text Solution
|