Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-Wave Nature of Matter-EXERCISE
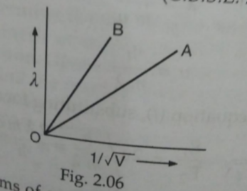
- The two lines A nad B in Fig.2.06 show the plot of de-Broglie waveleng...
Text Solution
|
- What is photons? Prove that its rest mass is zero.
Text Solution
|
- Are matter waves electromagnetic?
Text Solution
|
- Write de Brogile hypothesis for matter wave and find an expression fo...
Text Solution
|
- Write de Brogile hypothesis for matter wave and find an expression fo...
Text Solution
|
- What do you men by dual nature of matter ?
Text Solution
|
- Derive de Broglie’s equation.
Text Solution
|
- Calculate the de-Brogile wavelength of an electron.
Text Solution
|
- Find the de Brogile wavelength associated with an electron accelerated...
Text Solution
|
- Show that the de-Broglie wavelength lambda of electrons of energy E is...
Text Solution
|
- Derive the expression for de Broglie wavelength associated with an ele...
Text Solution
|
- Show that de-Broglie hypothesis of matter wave supports the Bohr's con...
Text Solution
|
- Derive the expression for de Broglie wavelength associated with an ele...
Text Solution
|
- Explain the dual behaviour of matter.
Text Solution
|
- Describe an experiment which shows the wave nature of electron.
Text Solution
|
- Calculate the wavelength of matter waves associated with a particles o...
Text Solution
|
- Deterine de-Brogile wavelength associated with a ball of mass 150 g tr...
Text Solution
|
- Find de Broglie wavelength of wave associated with a particle of rest ...
Text Solution
|
- Is it possible to observe de -Broglie wave associated with a material ...
Text Solution
|
- the de-Brolie wavelength of an electron is 2 overset @A.Calculate its ...
Text Solution
|
- Find the wavelength for a beam of neutrons,whose kinetic energy is 100...
Text Solution
|
 .
.