Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-STRUCTURE OF ATOM-EXERCISE
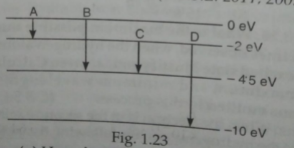
- The energy levels of an atom are as shown in Fig.1.23 Which one of the...
Text Solution
|
- Explain Rutherford’s experiment on the scattering of alpha particles f...
Text Solution
|
- What were the observations and important calculations drawn from a-sca...
Text Solution
|
- Discuss Rutherford’s experiment on the scattering of alpha particles.W...
Text Solution
|
- Discuss Rutherford’s experiment on the scattering of alpha particles.W...
Text Solution
|
- Write the main postulates of Rutherford’s atomic model and the cause o...
Text Solution
|
- Discuss in brief Rutherford's Model of atom.
Text Solution
|
- Write the main postulates of Rutherford’s atomic model and the cause o...
Text Solution
|
- Explain Rutherford’s model of the atom. What are its drawbacks ?
Text Solution
|
- Draw a labelled diagram of Geiger-Marsden experment on the scatttering...
Text Solution
|
- Describe the aloha particle scattering experiment for the discovery of...
Text Solution
|
- What are drawbacks of Rutherford’s atomic model ? How did Bohr remove ...
Text Solution
|
- What are drawbacks of Rutherford’s atomic model ? How did Bohr remove ...
Text Solution
|
- What are drawbacks of Rutherford’s atomic model ? How did Bohr remove ...
Text Solution
|
- Express 940 in roman numbers.
Text Solution
|
- Calculate the molecular mass of C12H22O11
Text Solution
|
- State Bohr’s postulates for atomic modeland using them derive an expre...
Text Solution
|
- Obtain an expression for energy of orbital electron in hydrogen atom u...
Text Solution
|
- Show that the radius of Bohr's orbit is directly proportional to the s...
Text Solution
|
- Express 941 in roman numbers.
Text Solution
|
- Express 942 in roman numbers.
Text Solution
|
 .
.