Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-STRUCTURE OF NUCLEUS-EXERCISE
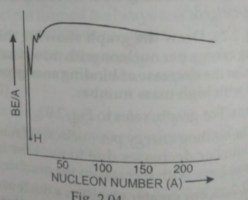
- The variation with nucleon number A of the finding energy per nucleon ...
Text Solution
|
- Define atomic number.
Text Solution
|
- Why is the denstiy of nucleus more than that of the atom?
Text Solution
|
- Explain any three properties of the nucleus.
Text Solution
|
- What it Einstein's mass energy relation?
Text Solution
|
- Define the atomic mass unit?
Text Solution
|
- Define the atomic mass unit?
Text Solution
|
- What are nuclear forces ? State their four properties.
Text Solution
|
- What do you mean by binding energy ? Explain the significance of bindi...
Text Solution
|
- What do you mean by binding energy ? Explain the significance of bindi...
Text Solution
|
- State and explain binding energy of a nucleus.
Text Solution
|
- Draw the graph showing variation of binding energy per nucleon with ma...
Text Solution
|
- Define binding energy, binding energy per nucleon. Draw and explain a ...
Text Solution
|
- State and explain mass defect and packing fraction.
Text Solution
|
- What is mass defect?
Text Solution
|
- With the help of example explain how the neutron-proton ratio changes ...
Text Solution
|
- Explain with the help of nuclear reaction in each of the following cas...
Text Solution
|
- Draw a plot of potential energy of a pair of nucleons as a function of...
Text Solution
|
- What are nuclear forces ? Discuss fourimportant properties of nuclear ...
Text Solution
|
- What are nuclear forces ? Discuss fourimportant properties of nuclear ...
Text Solution
|
- Draw a plot of potential energy of a pair of nucleons as a function of...
Text Solution
|
 .
.