A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-COORDINATION COMPOUNDS-EXERCISE
- Monel metal has the following composition-
Text Solution
|
- Match the coordination compounds given in Column I with the central me...
Text Solution
|
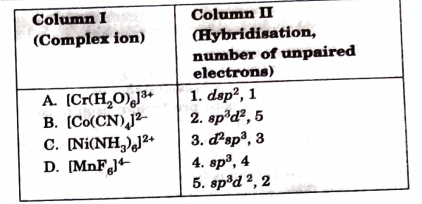
- Match the complex ions given in Column I with the hybridisation and nu...
Text Solution
|
- Complete the reaction
Text Solution
|
- Complete the reaction :
Text Solution
|
- In the following questions a statement of assertion followed by a stat...
Text Solution
|
- How many unpaired electrons are present in Gd(Z = 64) ?
Text Solution
|
- Why is geometrical isomerism not possible in tetrahedral compounds hav...
Text Solution
|
- Which type of complexes do not show geometrical isomerism ?
Text Solution
|
- In [Fe(H2O)6]^(3+), the magnetic moment corresponds to number of unpai...
Text Solution
|
- Give one example of linkage isomer.
Text Solution
|
- Why does NH3 readily form complexes but NH4^(+) does not ?
Text Solution
|
- Give evidence that [CO(NH3)5Cl]S04 and [CO(NH3)5(S04)]Cl are ionisati...
Text Solution
|
- Name central metal atom present in haemoglobin and Vitamin B12.
Text Solution
|
- Name one example of a hexadentate ligand.
Text Solution
|
- Write the IUPAC name of the following : K3[Fe (CN)5 NO]
Text Solution
|
- Write IUPAC name of the following : [Cr(NH3)3Cl3]
Text Solution
|
- Draw the structures of optical isomers of: [Cr(C2O4)3]^3-
Text Solution
|
- Draw the structures of optical isomers of: [Cr(C2O4)3]^3-
Text Solution
|
- The hexaquo manganese(II) ion contains five unpaired electrons, while ...
Text Solution
|