A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-HALOALKANES AND HALOARENES-EXERCISE
- In the addition of HBr to propene in the absence of Peroxides the firs...
Text Solution
|
- The major product in the reaction is CH3- underset (CH3) underset(|) ...
Text Solution
|
- In the reaction: A and B are respectively:
Text Solution
|
- Express 1731 in roman numbers.
Text Solution
|
- Express 1732 in roman numbers.
Text Solution
|
- Express 1733 in roman numbers.
Text Solution
|
- Iodoethane reacts with sodium in ether to form the product
Text Solution
|
- When ethyl iodide and n-propyl iodide aro allowed to react with sodium...
Text Solution
|
- Express 1735 in roman numbers.
Text Solution
|
- Express 1736 in roman numbers.
Text Solution
|
- Identify the set of reagents/reaction conditions ‘X’ and ‘Y’ in the fo...
Text Solution
|
- The intermediate during the addition of HCl to propene in the presence...
Text Solution
|
- Butane nitrile may be prepared by heating
Text Solution
|
- Express 1737 in roman numbers.
Text Solution
|
- Which of the following will be least reactive in nucleophilic substitu...
Text Solution
|
- Express 1738 in roman numbers.
Text Solution
|
- Express 1750 in roman numbers.
Text Solution
|
- Express 1751 in roman numbers.
Text Solution
|
- Express 1172 in roman numbers.
Text Solution
|
- Express 1753 in roman numbers.
Text Solution
|
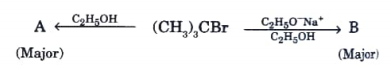 A and B are respectively:
A and B are respectively: