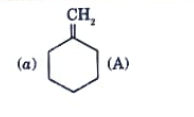
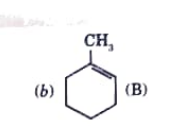
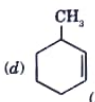
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-HALOALKANES AND HALOARENES-EXERCISE
- Discuss how gun metal is formed?
Text Solution
|
- Express 1765 in roman numbers.
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with the Mark...
Text Solution
|
- Out of SN1 and SN2, which reaction occurs with Racemisation.
Text Solution
|
- Discuss the formation of bell metal?
Text Solution
|
- Discuss the formation of constantan?
Text Solution
|
- The reaction of C6H5CH=CHCH3 with HBr produces
Text Solution
|
- Discuss how German silver is formed?
Text Solution
|
- For the following reactions: Which of the following statement is co...
Text Solution
|
- Discuss how Dutch metal is formed?
Text Solution
|
- Discuss how Hydroleum is formed?
Text Solution
|
- The hydrolysis of 2-bromo-3-methylbutane by SN1 mechanism gives mainly
Text Solution
|
- A dihalogen derivative (A) of a hydrocarbon having two carbon atoms re...
Text Solution
|
- When neopentyl bromide is subjected to Wurtz reaction the product form...
Text Solution
|
- Which one of the following gives only one monochlore derivative ?
Text Solution
|
- Arrange the following halides in order of increasing SN2 reactivity : ...
Text Solution
|
- In alkaline hydrolysis of a tertiary alkyl halide by aqueous alkali, i...
Text Solution
|
- Discuss how Aluminium bronze alloy is formed?
Text Solution
|
- The compound that does not undergo hydrolysis by SN1 mechanism is
Text Solution
|
- Discuss how nichrome alloy is formed?
Text Solution
|