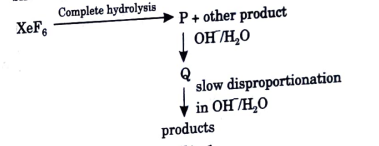
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-p-BLOCK ELEMENTS-EXERCISE
- Concentrated nitric acid, upon long standing, turns yellow- brown due ...
Text Solution
|
- The product formed in the reaction of SOCl2 with white phosphorus is
Text Solution
|
- Under ambient conditions, the total number of gases released as produc...
Text Solution
|
- White phosphorus has
Text Solution
|
- What happens when 14% of manganese combine with 80-85% of iron?
Text Solution
|
- Which of the following contains cationic iodine ?
Text Solution
|
- In its compounds, oxygen can show oxidation state of
Text Solution
|
- A solution of colourless salt H on boiling with excess NaOH produces...
Text Solution
|
- Nitrogen oxide(s) that contain(s) N-N bond(s) is (are)
Text Solution
|
- The correct statement(s) about O3 is (are)
Text Solution
|
- Ionization potential values of noble gases decrease down the group wit...
Text Solution
|
- The correct Statement(s) regarding, (i) HClO, (ii) HClO2, (iii) HClO3 ...
Text Solution
|
- The nitrogen containing compound produced in the reaction of HNO3 wit...
Text Solution
|
- Phosphorus forms a variety of oxyacids. In all these, phosphorus is sp...
Text Solution
|
- Write the IUPAC name of : [Fe (CO)5 ]
Text Solution
|
- Which of the acids show reducing properties ?
Text Solution
|
- Express 2556 in roman numbers.
Text Solution
|
- Which of these is not a tree?
Text Solution
|
- Which oxide of sulphur acts as oxidising as well as reducing agent?
Text Solution
|
- Which of the following statement is not correct ?
Text Solution
|