A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-p-BLOCK ELEMENTS-EXERCISE
- The correct Statement(s) regarding, (i) HClO, (ii) HClO2, (iii) HClO3 ...
Text Solution
|
- The nitrogen containing compound produced in the reaction of HNO3 wit...
Text Solution
|
- Phosphorus forms a variety of oxyacids. In all these, phosphorus is sp...
Text Solution
|
- Write the IUPAC name of : [Fe (CO)5 ]
Text Solution
|
- Which of the acids show reducing properties ?
Text Solution
|
- Express 2556 in roman numbers.
Text Solution
|
- Which of these is not a tree?
Text Solution
|
- Which oxide of sulphur acts as oxidising as well as reducing agent?
Text Solution
|
- Which of the following statement is not correct ?
Text Solution
|
- Haloalkanes are soluble in water.
Text Solution
|
- Among the following, the correct statement is
Text Solution
|
- White phosphorus on reaction with NaOH gives PH3 as one of the product...
Text Solution
|
- The reactions of Cl2 gas with cold-dilute and hot-concentrated NaOH in...
Text Solution
|
- Express 2555 in roman numbers.
Text Solution
|
- The questions given below consist of an Assertion and Reason. Use the ...
Text Solution
|
- Assertion : F2 has low reactivity. Reason: F-F bond has low bond dis...
Text Solution
|
- Assertion : F-F bond in F2 molecule is strong. Reason: F atom is smal...
Text Solution
|
- Assertion : P4 is more reactive than N2. Reason: P-P single bond in P4...
Text Solution
|
- The question given below consist of an assertion (A) and a reason ( R)...
Text Solution
|
- Explain, why HClO4 is stronger acid than HClO2
Text Solution
|
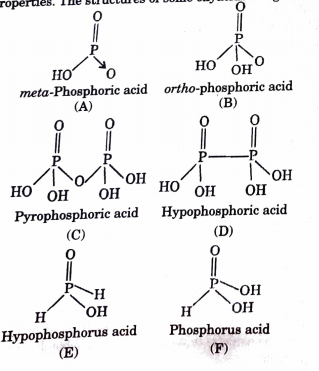
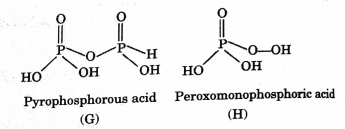 Answer the following (1 to 7) questions :
The oxyacid of P having tetrabasicity is
Answer the following (1 to 7) questions :
The oxyacid of P having tetrabasicity is