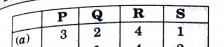
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-p-BLOCK ELEMENTS-EXERCISE
- Match each of the reactions given in Column I with the corresponding...
Text Solution
|
- The incomplete chemical reactions given in List I show missing reagent...
Text Solution
|
- Match the compound given in List I with structure and number of lone p...
Text Solution
|
- Write the IUPAC name of : K2[Fe (C2O4)3 ]
Text Solution
|
- Write the IUPAC name of : [Co (CN ) (en)2 (H2O) ]^(+2)
Text Solution
|
- Write the IUPAC name of : [Fe (CN)5 Br ]^(-3)
Text Solution
|
- Total number of dibasic acids among H3PO4, H4P2O7, H4P2O6, H3PO5, H3PO...
Text Solution
|
- Write the IUPAC name of : [Cr (NH3 )3 (en)2 ]Cl3
Text Solution
|
- The difference in the oxidation numbers of the two types of sulphur at...
Text Solution
|
- Reaction of Br2 with Na2CO3 in aqueous solution gives sodium bromide a...
Text Solution
|
- Among the following, the number of compounds that can react with PCl5 ...
Text Solution
|
- The difference between the number of lone pairs and P - O bonds in P4O...
Text Solution
|
- The total number of lone pairs of elections in N2O3 is
Text Solution
|
- On addition of conc. H2SO4 to a chloride salt, colourless fumes are ev...
Text Solution
|
- In qualitative analysis when H2S is passed through an aqueous solution...
Text Solution
|
- In a cyclotrimetaphosphoric acid molecule, how many single and double ...
Text Solution
|
- Which of the following elements can be involved in ppi -d pi bonding
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Affinity for hydrogen decreases in the group from fluorine to iodine. ...
Text Solution
|
- Bond dissociation enthalpy of E-H (E = element) bonds is given below. ...
Text Solution
|