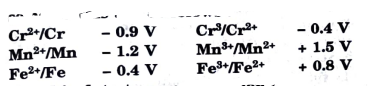Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-d-AND f- BLOCK ELEMENTS-EXERCISE
- For M^(2+)//M and M^(3+)//M^(2+) system the E^@ values for some metals...
Text Solution
|
- Name the third and fourth transition elements of first transition seri...
Text Solution
|
- What is the theoretical magnetic moment of Ti^(3+) ion ?
Text Solution
|
- Which of the two Zn(+2) or V(+4) is diamagnetic ?
Text Solution
|
- Which out of the following ions would form coloured complexes : Ni^(2+...
Text Solution
|
- How many unpaired electrons are present in each of the following ? Fe^...
Text Solution
|
- Out of V^(2+) and V^(3+) which is more paramagnetic and why ?
Text Solution
|
- Calculate the magnetic moment of Fe^(2+) ion (Z = 26).
Text Solution
|
- Name and write electronic configuration first three elements of second...
Text Solution
|
- Why Zn^(2+) salts are colourless and Ni2+ salts are coloured?
Text Solution
|
- A compound has been found to have magnetic moment of 3.9 B.M. How many...
Text Solution
|
- Name the catalyst of Vanadium used for oxidation of SO2 to SO3 in cont...
Text Solution
|
- Give reason, Mn^(2+) ion is more paramagnetic than Fe^(2+) ion.
Text Solution
|
- Express 1312 in roman numbers.
Text Solution
|
- Express 1313 in roman numbers.
Text Solution
|
- Express 1315 in roman numbers.
Text Solution
|
- Express 1320 in roman numbers.
Text Solution
|
- One day is equal to ............... seconds.
Text Solution
|
- Write ionic equation showing KMnO4 acting as an oxidising agent in aci...
Text Solution
|
- The oxidation state of chromium in dichromate ion (Cr2O7^(2-)) and chr...
Text Solution
|
- What is the oxidation state of Mn is manganate ion.
Text Solution
|