A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-COORDINATION COMPOUNDS-EXERCISE
- Give the formula of following compound : potassium tetrachloridopal...
Text Solution
|
- Express 2320 in roman numbers.
Text Solution
|
- An aqueous solution of metal ion M1 reacts separately with reagents Q ...
Text Solution
|
- Give the formula of following compound : diamminechloridonitrito-N-...
Text Solution
|
- Give the formula of following compound : pentaamminenitrito-N-cobal...
Text Solution
|
- Give the formula of following compound : pentaamminenitrito-O-cobal...
Text Solution
|
- Assertion : NF3 is weaker ligand than N(CH3)3). Reason : NF3 ionises t...
Text Solution
|
- Give the formula of following compound tetraamminechloridonitrito-N-pl...
Text Solution
|
- Assertion : [Ni(CO)4] is diamagnetic and tetrahedral in shape. Reason ...
Text Solution
|
- Give the formula of following compound : potassium trioxalatoaluminate...
Text Solution
|
- Give the formula of following compound : amminedichlorido(pyridine)pla...
Text Solution
|
- Give the formula of following compound : sodium amminebromidochloridon...
Text Solution
|
- Give the formula of following compound : triamminetrichloridochromium(...
Text Solution
|
- Give the formula of following compound : potassium hexacyanidoferrate(...
Text Solution
|
- Give the formula of following compound : sodium hexaflouridoaluminate(...
Text Solution
|
- Give the formula of following compound : tris (ethylene diamine) cobal...
Text Solution
|
- Alanko is a mixture of different metals. Explain this statement and on...
Text Solution
|
- Is the iupac name of compound is correct ? K2[PtCl4] potassium tetrac...
Text Solution
|
- Manganese steel is an alloy and is made of different metals. Explain t...
Text Solution
|
- Is the iupac name of compound is correct ? Na2[Ni EDTA] sodium ethyle...
Text Solution
|
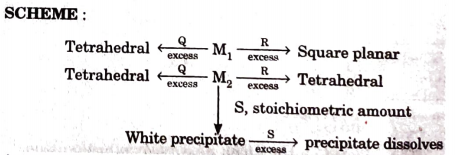 `M1, Q and R`, respectively are :
`M1, Q and R`, respectively are :