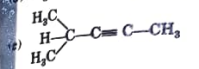
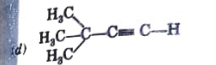
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS-EXERCISE
- Choose the correct statement
Text Solution
|
- Show that every positive even integer is of the form 2q, for some inte...
Text Solution
|
- An acyclic hydrocarbon P, having molecular formula C6H10, gave acetone...
Text Solution
|
- The structure of the compound Q is
Text Solution
|
- In the following reaction sequence, the compound J is intermediate. I ...
Text Solution
|
- The correct order of reactivity of the following derivatives of carbox...
Text Solution
|
- Mass cannot be converted into energy.(True/ False)
Text Solution
|
- What happens when methyl chloride is treated with KCN ?
Text Solution
|
- Define the terms ' average rate ' and 'instantaneous rate'?
Text Solution
|
- Which is correct statement ?
Text Solution
|
- Give chemical equation for the following conversion : Cyclohexanol to ...
Text Solution
|
- Assertion : In methanal, all the four atoms are in the same plane. Rea...
Text Solution
|
- Assertion : Benzaldehyde is more reactive than propanal towards nucleo...
Text Solution
|
- Assertion: Acetaldehyde undergoes aldol condensation with dil. NaOH. R...
Text Solution
|
- Would you expect Benzaldehyde to be more reactive in nucleophile addit...
Text Solution
|
- Give chemical reaction of Reimer-Tiemann Reaction?
Text Solution
|
- Assertion : The pKa of acetic acid is lower than that of phenol. Reas...
Text Solution
|
- Assertion : Benzoic acid and phenol can be distinguished by NaHCO3. Re...
Text Solution
|
- Assertion : Fluoroacetic acid is stronger acid than chloroacetic acid....
Text Solution
|
- Assertion : Ethanoic acid liberates hydrogen with sodium metal. Reaso...
Text Solution
|
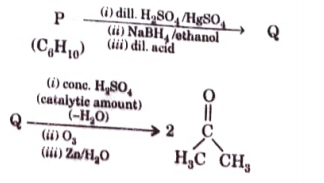 The structure of compound P is
The structure of compound P is