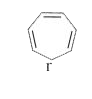
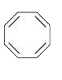
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-SOLVED PAPER 2017 -PART II (CHEMISTRY)
- The night-blindness is developed due to deficiency of vitamin
Text Solution
|
- The transfer RNA anticodon for the messenger RNA codon G-C-A is
Text Solution
|
- If 0.765 g of an acid gives 0.535 g of CO(2) and 0.138 g of H(2)O, the...
Text Solution
|
- Maximum pK(b) value is of
Text Solution
|
- Which of the following is an incorrect set of quantum members?
Text Solution
|
- The most acidic oxide for nitrogen is
Text Solution
|
- Which of the following show maximum bond-order?
Text Solution
|
- Which of the following show an increase in entropy? I Boiling of wa...
Text Solution
|
- BF(3) is an acid , according to
Text Solution
|
- For the reaction N(2)O(4)(g)to2NO(2)(g)
Text Solution
|
- Which of the following elements mostly form covalent compounds?
Text Solution
|
- When aqueous solutions of borax is acidified with HCl, we get
Text Solution
|
- Which of the following compound does not follow Huckel’s rule?
Text Solution
|
- A graph is ploted between log K virsus (1)/(T) for calculation of acti...
Text Solution
|
- Hybridization of Fe in K(3)Fe(CN)(6) is
Text Solution
|
- Which of the following shows maximum magnetic moment?
Text Solution
|
- Consider the following radioactive decays I. ""(92)Uoverset(-alpha)...
Text Solution
|
- Chlorobenzene overset("Reaction")underset(X)rarr "Phenol" overset("Rea...
Text Solution
|
- Which of the following has largest number of moles ?
Text Solution
|
- One moles each of four ideal gases are kept as follows. I. 5 L of ...
Text Solution
|