A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-SOLVED PAPER 2019 BITSAT-PART -II ( CHEMISTRY )
- The chemical reaction 2O(3)rarr3O(2) proceeds as follows : O(3)rarrO...
Text Solution
|
- An aqueous solution freezes at 272.4 K, while pure water freezes at 27...
Text Solution
|
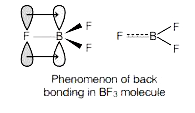
- The order of acidic strenght boron trihalides is:
Text Solution
|
- The correct order of mobility of alkali metal ions in aqueous solution...
Text Solution
|
- Thermosetting polymer, Bakeline is formed by the reaction of phenol w...
Text Solution
|
- A drug that is antipyretic as well as analgesic is :
Text Solution
|
- The correct statement about the following disaccharide is–
Text Solution
|
- If one strand of DNA has the sequence ATCGTATG , the sequence in the c...
Text Solution
|
- Which of the following salt would give SO2 with hot and dil. H2 SO4 an...
Text Solution
|
- In the reaction (X) and (Y) are respectively.
Text Solution
|
- Which of the following reaction is an example of use of water gas in t...
Text Solution
|
- Which of the following compounds, on reaction with NaOH and Na(2)O(2) ...
Text Solution
|
- For the reaction– rate of reaction is faster, when Z is–
Text Solution
|
- E(1), E(2) and E(3) are the emfs of the following three galvanic cells...
Text Solution
|
- Sodium nitroprusside when added to an alkaline solution of sulphide io...
Text Solution
|
- For a reverse reaction, A rarr B, which one of the following statement...
Text Solution
|
- Which of the following reactants is used for the preparation of ethyl ...
Text Solution
|
- Given pH of a solution A is 3 and it is mixed with another solution B ...
Text Solution
|
- In freundlich adsorption isotherm, the value of 1/n is :
Text Solution
|
- The spin only magnetic moment of Mn^(4+) ion is nearly
Text Solution
|