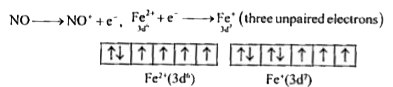
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE D-AND F-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-II)|50 VideosTHE D- AND F-BLOCK ELEMENT
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|40 VideosTHE P-BLOCK ELEMENTS (XII)
BRILLIANT PUBLICATION|Exercise LEVEL- II (GROUP 18 NOBLE GASES) (ASSERTION - REASON) |5 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-THE D-AND F-BLOCK ELEMENTS-QUESTIONS (LEVEL-III)
- Consider following statements, I. The size of the lanthanide M^(3+) io...
Text Solution
|
- Most transition metals I:forms sets of compounds which display differe...
Text Solution
|
- [Fe(H2 O)5 NO]^(2+) is brown-ring complex. In this complex
Text Solution
|
- When K2 CrO4 is added to CuSO4 solution, there is formation of CuCrO4...
Text Solution
|
- Select the correct statements(s)
Text Solution
|
- Which of the following statements are true?
Text Solution
|
- Although +3 is the characteristic oxidation state for lanthanoids, cer...
Text Solution
|
- Select correct statements.
Text Solution
|
- When CO2 is passed into aqueous
Text Solution
|
- Select correct statements (s)
Text Solution
|
- Which of the following statements are correct
Text Solution
|
- Amongst the following, identify the species with an atom in +6 oxidati...
Text Solution
|
- Which metals are present in german silver?
Text Solution
|
- The complex forming tendency of a transition metal depends upon:
Text Solution
|
- The correct statements about Cr^(2+) and Mn^(3+) is (are) (Atomic numb...
Text Solution
|
- Which of the following salt solutions will be coloured?
Text Solution
|
- Potassium manganate (K2 MnO4) is formed when
Text Solution
|
- The magnetic moment of a metal ion of first transition series is 2.83 ...
Text Solution
|
- The coefficient of H2 SO4 on balancing the following equation is .......
Text Solution
|
- The number of electrons present in the 5f orbital in ground state of N...
Text Solution
|