Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
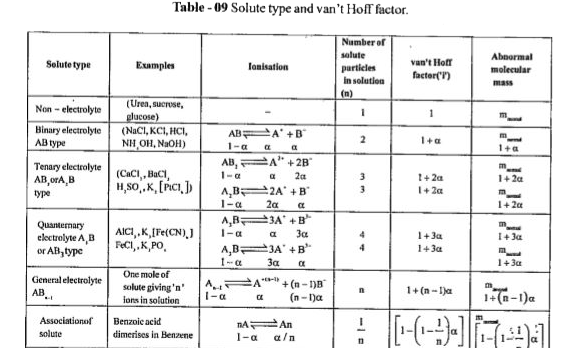
- If alpha=50%" for "K(4) [Fe(CN)(6)], find out van't Hoff factor 'I'.
Text Solution
|
- The degree of dissociation for K(4)[Fe(CN)(6)] is 60%. Calculate the V...
Text Solution
|
- Which one of the following salts will have the same value of van't hof...
Text Solution
|
- If alpha = 50% for K(4)[Fe(CN)(6)] then find Van't hoff factor 'i'
Text Solution
|
- 0.1 M K(4)[Fe(CN)(6)] is 50% ionised in aqueous medium. What will be i...
Text Solution
|
- The Van't Hoff factor (i) for a dilute solution of K(3)[Fe(CN)(6)] is
Text Solution
|
- Which of the salts has the same value of van't Hoff factor 'i' as K(3)...
Text Solution
|
- Van't Hoff factor of centimolal solution of K(3) [Fe(CN)(6)] i...
Text Solution
|
- Which onc of the following salts will have the same value of van't Hof...
Text Solution
|