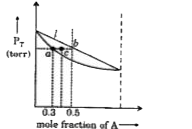
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-SOLUTIONS-Level-III (Linked Comprehension Type)
- 1 mole of A and 1 mole B are mixed together to form an ideal solution ...
Text Solution
|
- 1 mole of A and 1 mole B are mixed together to form an ideal solution ...
Text Solution
|
- 1 mole of A and 1 mole B are mixed together to form an ideal solution ...
Text Solution
|
- A substance trimerises when dissovled in a solvent A. The van't Hoff f...
Text Solution
|
- 0.1M K(4)[Fe(CN)(6)] is 60% ionized. What will be its van't Hoff facto...
Text Solution
|
- The molar mass of the solute sodium hydroxide obtained from the measur...
Text Solution
|
- The osmotic pressure pi depends on the molar concentration of the solu...
Text Solution
|
- The osmotic pressure pi depends on the molar concentration of the solu...
Text Solution
|
- The osmotic pressure pi depends on the molar concentration of the solu...
Text Solution
|
- The vapour pressure of two pure liquids A and B which form an ideal so...
Text Solution
|
- The vapour pressure of two pure liquids A and B which form an ideal so...
Text Solution
|
- The vapour pressure of two pure liquids A and B which form an ideal so...
Text Solution
|