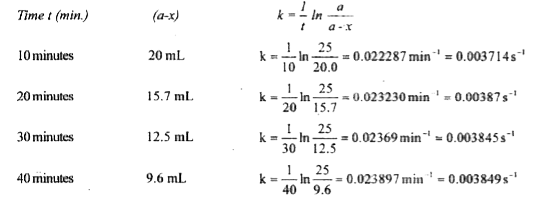
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- From the following data show that the decomposition of hydrogen pero...
Text Solution
|
- The following data represent for the decomposition of NH(4)NO(2) in aq...
Text Solution
|
- Decomposition of H(2)O(2) is a first-order reaction. A solution of H(2...
Text Solution
|
- From the following data, show that the decomposition of hydrogen perox...
Text Solution
|
- From the following data, show that the decomposition of hydrogen perox...
Text Solution
|
- To calculate the rate of decomposition of H(2)O(2) in an aqueous sol...
Text Solution
|
- From the following data show that decomposition of H(2)O(2) in aqueous...
Text Solution
|
- (a) (i) From the following data, show that the decomposition of hydrog...
Text Solution
|
- From the following data , show that the decomposition of hydrogen pero...
Text Solution
|