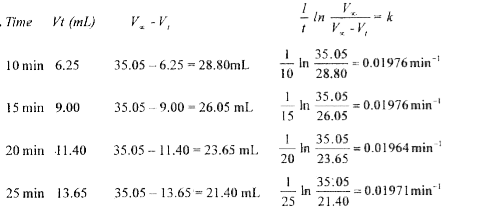Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- From the following data for the decomposition of ammonium nitrite in ...
Text Solution
|
- The following data represent for the decomposition of NH(4)NO(2) in aq...
Text Solution
|
- The decomposition of an aqeous solution of ammonium nitrite was studie...
Text Solution
|
- The following data are for the decomposition of ammonium nitrite in aq...
Text Solution
|
- 48^(@) C ताप पर (C)Cl(4) में N(2)O(5) के विघटन में निम्न उपादेय प्राप्...
Text Solution
|
- form the following data for the decompoistion of N(2)O(5) in carbon te...
Text Solution
|
- From the following data, show that the decomposition of hydrogen perox...
Text Solution
|
- निम्न आँकड़ो के आधार पर सिद्ध कीजिये कि अमोनियम नाइट्रेट का जलीय विलयन ...
Text Solution
|
- The decomposition of ammonium nitrite was studied by placing the appar...
Text Solution
|