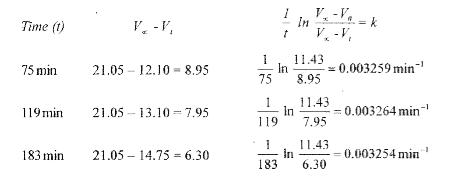Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- 3.5 ml ethyl acetate was added to a flask containing 100 ml of 0.1 M ...
Text Solution
|
- 500 " mL of " 1.0 M H2C2O4, 100 " mL of " 2.0 M H2SO4, and 40 g of NaO...
Text Solution
|
- 1.0 mL of ethyl acetate was added to 25 mL of N//2 HCl, 2 mL of the mi...
Text Solution
|
- In the titration of 25.0 mL of 0.1 M aqueous acetic acid (K(a) = 1.8 x...
Text Solution
|
- Following solutions were prepared by mixing different volumes of NaOH ...
Text Solution
|
- Following solutions were prepared by mixing different volumes of NaOH ...
Text Solution
|
- 30^(@) C ताप पर 0.1 N HCl में 5 mL इथील एसिटेट मिलाया गया। विभिन्न समय...
Text Solution
|
- 1.0 ml of ethyl acetate was added to 25 ml of N/2 HCl. 2 ml of the mix...
Text Solution
|
- Following solutions were prepared by mixing different volumes of NAOH ...
Text Solution
|