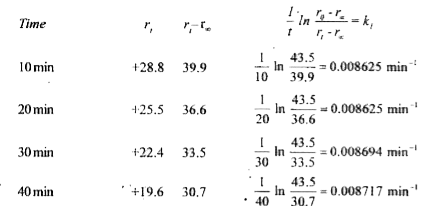Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The optical rotation of surcose in 0.5 M HCI at 35^(@)C at various ...
Text Solution
|
- Write the modal class for the following frequency distribution: Class-...
Text Solution
|
- The first order reaction: Sucrose rarr Glucose + Fructose takes place ...
Text Solution
|
- The time taken for 10 % completion of a first order reaction is 20 min...
Text Solution
|
- Optical rotation of sucrose in 1 N Hcl at various times was found as s...
Text Solution
|
- एक प्रथम कोटि की अभिक्रिया का अर्ध-आयु काल 10 मिनट है यदि अभिक्रिया प्...
Text Solution
|
- 6 गेंदों को 2, 4, 6, 8, 10, 12 मिनट के अंतराल पर क्रमश: घुमाया जाता है...
Text Solution
|
- Consider the following first order reaction taking place at 308 K in 0...
Text Solution
|
- An airplane propeller rotates 1000 times per minute. Find the number o...
Text Solution
|