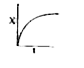
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- For a I order reaction which one is not correct?
Text Solution
|
- Which one the following statement for order of reactions is not correc...
Text Solution
|
- Which one the following statement for order of reactions is not correc...
Text Solution
|
- Which of the following statements is correct regarding order of reacti...
Text Solution
|
- Which of the following relation is correct for a first order reaction?...
Text Solution
|
- Which one of the following statements for order of reaction is not cor...
Text Solution
|
- अभिक्रिया की कोटि के लिए निम्न में से कौन-सा कथन सही नहीं है?
Text Solution
|
- Which one is correct for first order reaction.
Text Solution
|
- Which one of the following statement for order of reaction is not corr...
Text Solution
|