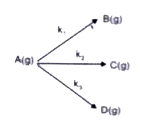
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A gaseous compound A reacts by three independent first order processes...
Text Solution
|
- A gaseous mixture contains three gaseous A,B and C with a total number...
Text Solution
|
- At 300 K the half-life of a sample of a gaseous compound initially at ...
Text Solution
|
- A gaseous compound A reacts by three independent first order process (...
Text Solution
|
- The reaction given below is observed to be first order with rate const...
Text Solution
|
- At 300 k the half - life a sample of a gaseous compound initially at 1...
Text Solution
|
- The reaction given below is observed to be first order with rate const...
Text Solution
|
- A gaseous mixture contains three gaseous A,B and C with a total number...
Text Solution
|
- At 300 K the half-life of a sample of a gaseous compound initially at ...
Text Solution
|