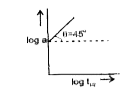
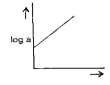
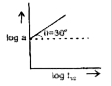
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following graphs is correct for the following reaction? ...
Text Solution
|
- Given the IUPAC names of the following compounds: (i) CH(3)CH(2)CH(2)u...
Text Solution
|
- Write IUPAC name of the following compound (a) CH(3)-CH(2)-underset(CH...
Text Solution
|
- Write the IUPAc name of the following compound : (a) CH(3)-CH(2)-ov...
Text Solution
|
- Give the IUPAC names of the following compounds: (i) C(6)H(5)-CH(2)-...
Text Solution
|
- Which of the following graphs is correct for the following reaction? ...
Text Solution
|
- The IUPAC name of the compound CH(3)CH(2)CH(2)underset(CH(CH(3))(2))un...
Text Solution
|
- Write down the IUPAC names of the following compounds :- (A) CH(3)-und...
Text Solution
|
- Which is correct IUPAC name of the following compound. CH(3)-overs...
Text Solution
|