A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
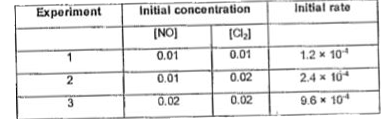
- For the reaction, 2NO + Cl(2) rarr 2NOCl at 300 K, following data are ...
Text Solution
|
- For the reaction 2NOCl(g) hArr 2NO(g)+Cl(2)(g) , the equilibrium const...
Text Solution
|
- Mechanism of the reaction 2NO + Cl(2) rarr 2NOCl may be written as 2NO...
Text Solution
|
- For a reaction, Cl(2)(g) + 2NO(g) to 2NOCl(g) ,the rate law is express...
Text Solution
|
- For the reaction 2NOrarr2NOCl at 300 K, following data are obtained: W...
Text Solution
|
- For a reaction 2NO(g) +Cl(2)(g) rarr 2NOCl(g) , when concentration of ...
Text Solution
|
- The rate of reaction : 2NO+Cl(2) to 2NOCl is given by the rate equat...
Text Solution
|
- The reaction between NO and Cl(2) written as 2NO +Cl(2) rarr ...
Text Solution
|
- For the reaction Cl(2)(g)+2NO(g) to 2NOCl (g) , the rate law is expres...
Text Solution
|