Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ACID, BASES AND SALTS-EXERCISE
- The following will have pH more than 7 or less than 7? Bee sting
Text Solution
|
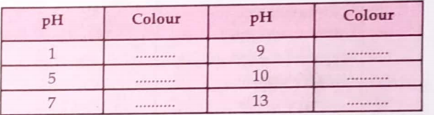
- Complete the following table
Text Solution
|
- Write the approximate colour of the universal indicator with the solut...
Text Solution
|
- The following data about the pH of different solutions are given: Whi...
Text Solution
|
- The following data about the pH of different solutions are given: Whi...
Text Solution
|
- The following data about the pH of different solutions are given: Whi...
Text Solution
|
- The following data about the pH of different solutions are given: Whi...
Text Solution
|
- The following data about the pH of different solutions are given: Wil...
Text Solution
|
- Select the substance from the following list which turn blue litmus so...
Text Solution
|
- What do you call the property of losing water of crystallisation?
Text Solution
|
- Fill ups The chemical formula of soda ash is………………. .
Text Solution
|
- Common name of Na2 CO3. 10H2O
Text Solution
|
- Fill ups Brine is a saturated solution of………………
Text Solution
|
- Write the chemical name and formula of baking soda?
Text Solution
|
- Write the formula of sodium carbonate and also state whether its water...
Text Solution
|
- Name the sodium compound which is used for softening hard water.
Text Solution
|
- Give one example each of normal salt?
Text Solution
|
- Give one example each of acidic salt.
Text Solution
|
- Give one example each of basic salt.
Text Solution
|
- Name the acid and the base which from the salts: Ammonium chloride.
Text Solution
|