Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ACID, BASES AND SALTS-EXERCISE
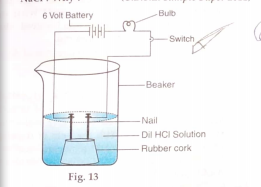
- An appratus was set up as shown in the figure. It was observed that wh...
Text Solution
|
- Name the gas produced when Sodium carbonate reacts with hydrochloric a...
Text Solution
|
- Name the gas produced when Magnesium carbonate reacts with hydrochlori...
Text Solution
|
- Name an indicator which is red in acid solution but turns blue in basi...
Text Solution
|
- Name one strong and one weak acid.
Text Solution
|
- Why is cold milk helps a person in neutralising acidity in the stomach...
Text Solution
|
- Give the name and formula of acid present in vinegar?
Text Solution
|
- Name the acids present in Orange?
Text Solution
|
- Name the acids present in Lemon?
Text Solution
|
- Name the acids present in Tomatoes.
Text Solution
|
- The pH of fresh milk is 6. will its pH value increase or decrease when...
Text Solution
|
- An acidic solution contains……………..ions.
Text Solution
|
- A basic solution contains……………..ions.
Text Solution
|
- Name two acid base indicators?
Text Solution
|
- The pH values of some substances are given below:(i)Apples: 5.0-6.5(ii...
Text Solution
|
- What is the pH of an acid having [H^+]=10^-3M
Text Solution
|
- Will the OH^- ions concentration increase or decrease if a 1M NaOH sol...
Text Solution
|
- Two solutions A and B have pH of 6 and 9 respectively. Which solution ...
Text Solution
|
- Four test tubes A,B,C and D contain solutions of pH 3.0, 5.0, 6.0 and ...
Text Solution
|
- Four test tubes A,B,C and D contain solutions of pH 3.0, 5.0, 6.0 and ...
Text Solution
|
- Which of the following have large pH values: 1M CH3COOH or 1M HCl
Text Solution
|