Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS-EXAMPLE
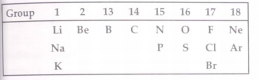
- A part of the periodic table is show below: Which of these is/are nob...
Text Solution
|
- A part of the periodic table is show below: Which of these is/are hav...
Text Solution
|
- A part of the periodic table is show below: Which of these is/are hav...
Text Solution
|
- A part of the periodic table is show below: Which of these is/are alk...
Text Solution
|
- Consider the group 1 of the periodic table and answer the following qu...
Text Solution
|
- Consider the group 1 of the periodic table and answer the following qu...
Text Solution
|
- Consider the group 1 of the periodic table and answer the following qu...
Text Solution
|
- Consider the group 1 of the periodic table and answer the following qu...
Text Solution
|
- Two elements X and Y have atomic number 6 and 17 respectively. To whic...
Text Solution
|
- The second period of the long time of periodic table contains the foll...
Text Solution
|
- The second period of the long time of periodic table contains the foll...
Text Solution
|
- How were the positions of isotopes of varoius elements decided in the ...
Text Solution
|
- Is it possible to have an element with atomic number 1.5 placed betwee...
Text Solution
|
- Where do you think should hydrogen be placed in the Modern Peridic tab...
Text Solution
|
- How do you calculate the valency of an element from its electronic con...
Text Solution
|
- What is the valency of magnesium with atomic number 12 and sulphur wit...
Text Solution
|
- Find out the valencies of the first two elements.
Text Solution
|
- How does the valency vary in the period on going from left to right?
Text Solution
|
- How does the valency vary on going down a group?
Text Solution
|
- In a periodic table How do you think the tendency to lose electrons wi...
Text Solution
|