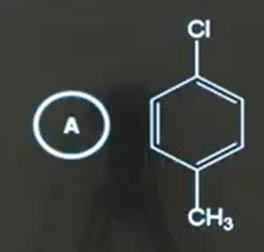
A
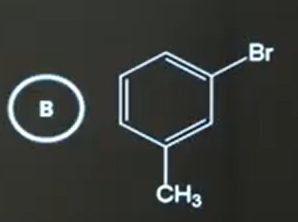
B
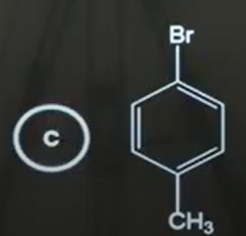
C
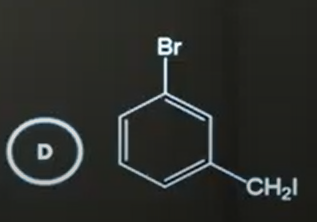
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following reactions gives yellow precipitate for the foll...
Text Solution
|
- Which of the following ketone will not give yellow precipitate with Na...
Text Solution
|
- Which of the following will give yellow precipitate with I(2)//NaOH -
Text Solution
|
- Which of the following will give yellow precipitate on shaking with an...
Text Solution
|
- Which of the following will not give yellow precipitate when treated w...
Text Solution
|
- Which of the following will give yellow precipitate on shaking with an...
Text Solution
|
- Which of the following will give yellow precipitate with I(2)//NaOH-
Text Solution
|
- Which of the following will give yellow precipitate with l(2)//NaOH ?
Text Solution
|
- Which of the following will give yellow precipitate with l(2)//NaOH ?
Text Solution
|