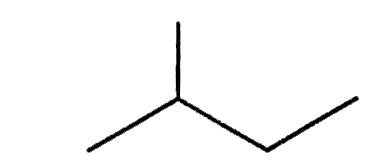
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A hydrocarbon of molecular weight 72gmol^(-1) has a 2-methyl group. Wh...
Text Solution
|
- यौगिकों के IUPAC नाम लिखिए - ऐल्केन (Alkanes) संरचना सूत्र (IUPAC ना...
Text Solution
|
- यौगिकों के IUPAC नाम लिखिए - ऐल्केन (Alkanes) संरचना सूत्र (IUPAC ना...
Text Solution
|
- यौगिकों के IUPAC नाम लिखिए - ऐल्केन (Alkanes) संरचना सूत्र (IUPAC ना...
Text Solution
|
- यौगिकों के IUPAC नाम लिखिए - ऐल्केन (Alkanes) संरचना सूत्र (IUPAC ना...
Text Solution
|
- यौगिकों के IUPAC नाम लिखिए - ऐल्कीन (Alkenes) संरचना सूत्र (IUPAC ना...
Text Solution
|
- यौगिकों के IUPAC नाम लिखिए - ऐल्कीन (Alkenes) संरचना सूत्र (IUPAC ना...
Text Solution
|
- यौगिकों के IUPAC नाम लिखिए - ऐल्कीन (Alkenes) संरचना सूत्र (IUPAC ना...
Text Solution
|
- यौगिकों के IUPAC नाम लिखिए - ऐल्कीन (Alkenes) संरचना सूत्र (IUPAC ना...
Text Solution
|