Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
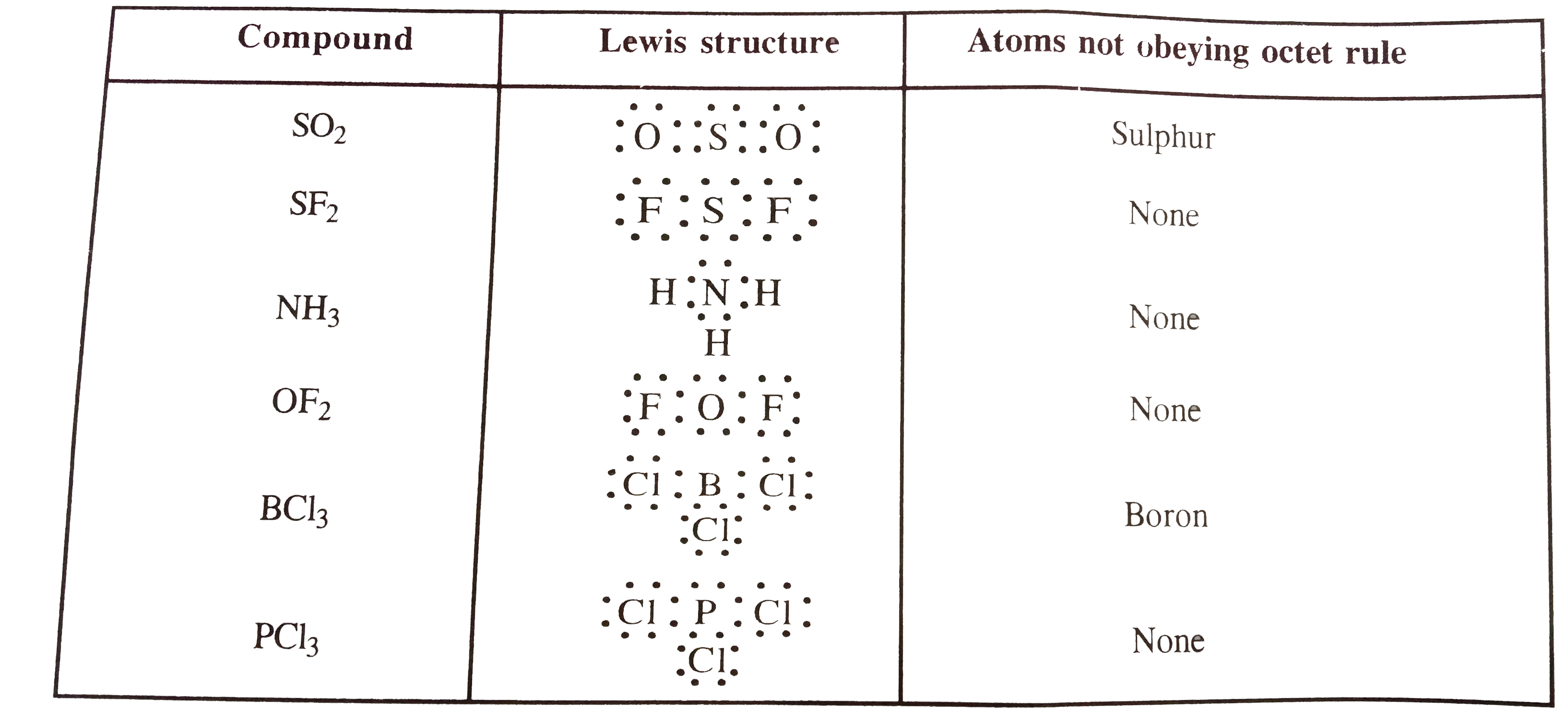
- Identify the atoms in each of the following compounds which do not obe...
Text Solution
|
- Identify the atoms in each of the following compounds which do not obe...
Text Solution
|
- In which one of the following compounds does the central atom obey the...
Text Solution
|
- In which of the following compounds does not central atom obey the oct...
Text Solution
|
- In which of the following compounds does the central atom obey the oct...
Text Solution
|
- In which of the following compounds does the central atom obey the oct...
Text Solution
|
- The number of lone pairs on S atom in SF2, SF4 and SF6 are respectivel...
Text Solution
|
- SF2,SF4 तथा SF6 में सल्फर परमाणु पर संकरण क्रमशः इस प्रकार होता है
Text Solution
|
- BF3, NCl3 , H2S , SF4 तथा BeCl2 यौगिकों में किसी एक केन्द्रीय परमाणु स...
Text Solution
|