Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
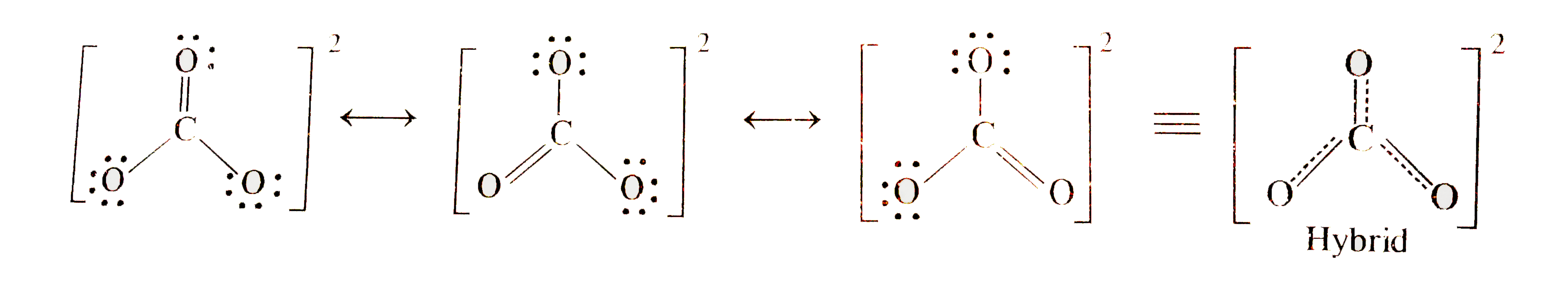
- Write the resonance structure of CO3^(2-) ion.
Text Solution
|
- CO3^(2-) ion exists as resonance hybrid of three equivalent structures...
Text Solution
|
- फिनॉक्साइड आयन की अनुनादी संरचनाऐं लिखिए ।
Text Solution
|
- Write the resonance structures of CO3^(2-) and HCO3^(-) .
Text Solution
|
- ClO4 ^ - Write the resonating structures of ions.
Text Solution
|
- How many resonating Structures are these for CO3^(2-) ion?
Text Solution
|
- How many resonating Structures are these for CO3^(2-) ion?
Text Solution
|
- How many resonating Structures are these for CO3^(2-) ion?
Text Solution
|
- Write resonance structures of benzee diazonium ion
Text Solution
|