Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
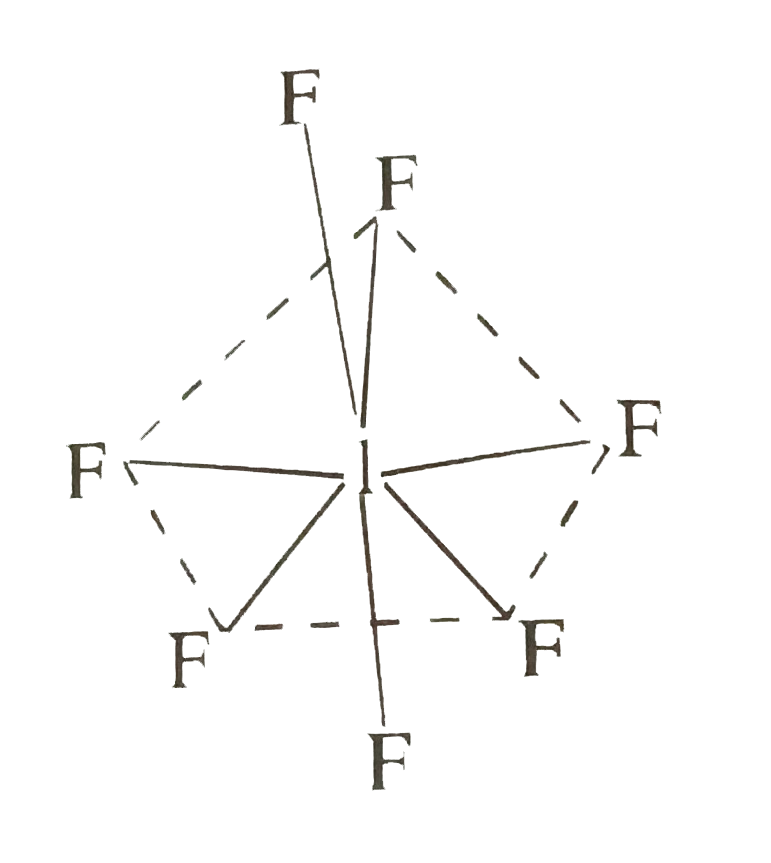
- Predict the shape on the basis of VSEPR theory. IF7
Text Solution
|
- On the basis of VSEPR theory predict the shape of the Ozone .
Text Solution
|
- Applying VSEPR theory, how to predict the shape of IF7?
Text Solution
|
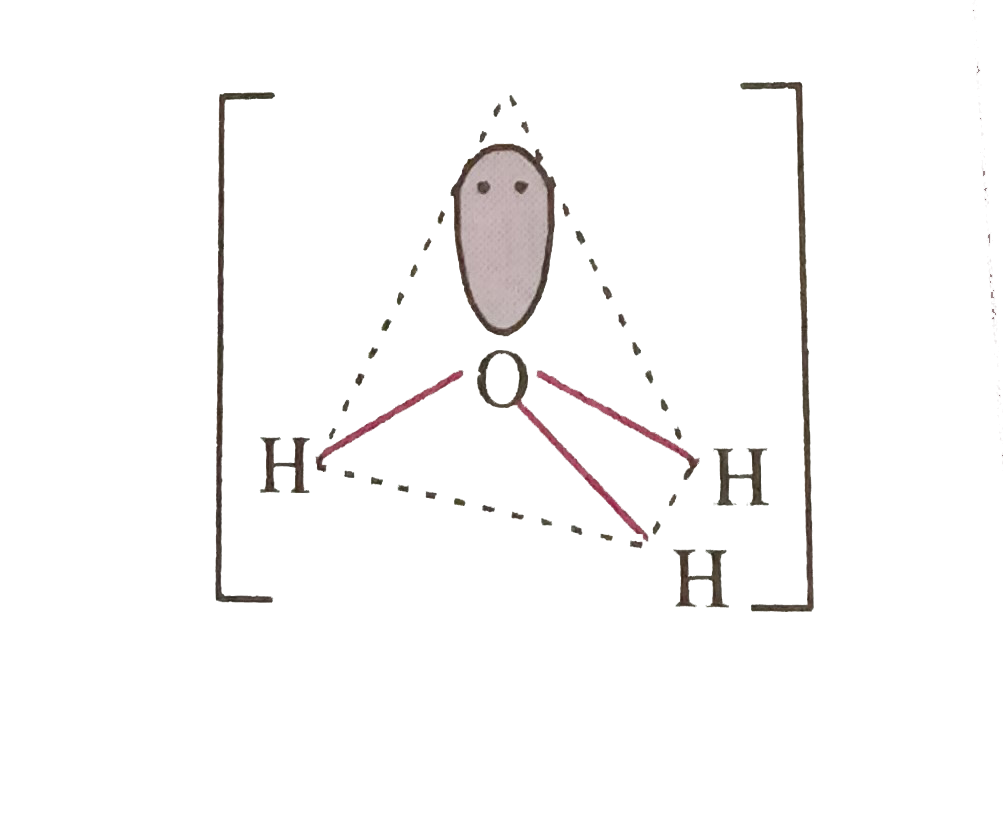
- VSEPR theory is used to predict the shape of covalent molecules . b) ...
Text Solution
|
- VSEPR theory is used to predict the shape of covalent molecules.Based ...
Text Solution
|
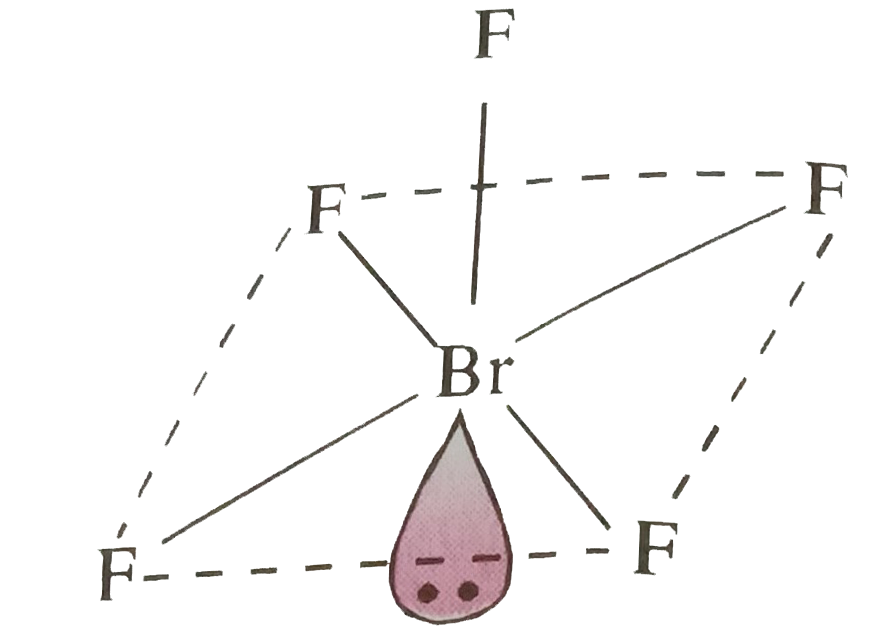
- Give the shapes of CIF3, BrF5 and IF7 using VSEPR theory.
Text Solution
|
- Predict the shape on the basis of VSEPR theory. BeCl2
Text Solution
|
- Predict the shape on the basis of VSEPR theory. BF3
Text Solution
|
- Predict the shape on the basis of VSEPR theory. PF5
Text Solution
|