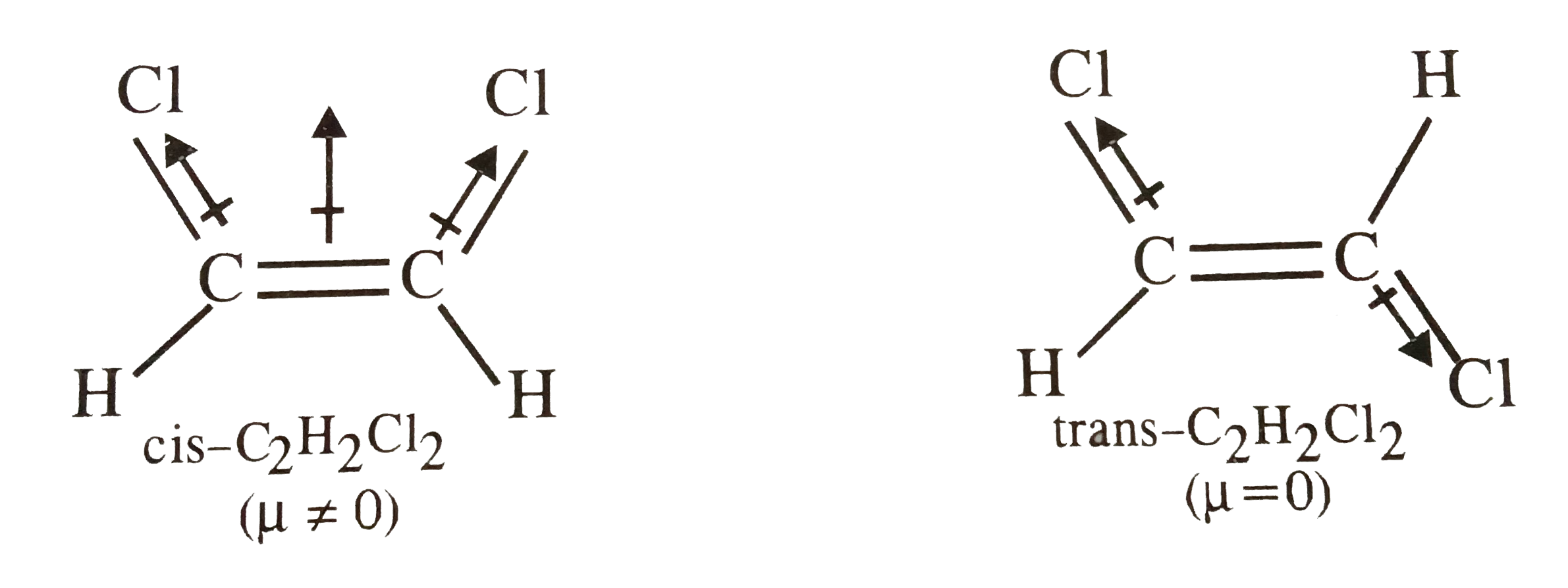Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Sketch the bond moments and resultant dipole moments in cis and trans ...
Text Solution
|
- Assertion:- Boiling point of cis-2-butene is more than trans-2-butene....
Text Solution
|
- Dipole moment of cis-2, 3-dichloro-2 butne is ….. Than the dipole mome...
Text Solution
|
- Statement-1: Dipole moment of H(2)O is more than that of OF(2) Stateme...
Text Solution
|
- Respresent diagrammatically the bond moments and the resultant dipole ...
Text Solution
|
- Dipole moment will be zero in the complexes I. [Ni(CN)(4)]^(2-) II...
Text Solution
|
- Which has the least dipole moment-1 -butene, cis-2-butene, trans-2-but...
Text Solution
|
- Statement-1 : Dipole moment of H(2)O is more than that of OF(2) . Stat...
Text Solution
|
- The boiling poing (in K) of cis but -2- ene and dipole moment ( in D) ...
Text Solution
|